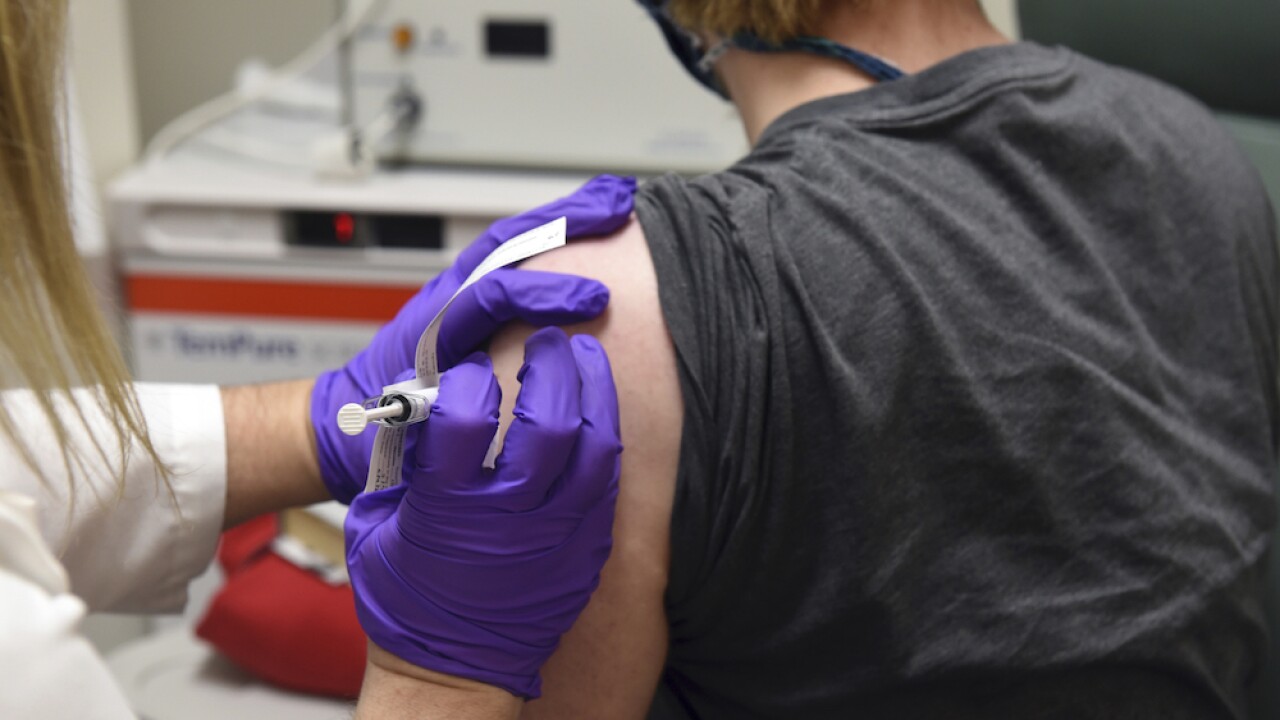
Top officials tapped with developing, approving and distributing COVID-19 vaccines say they're on track to begin distributing the first doses in the coming weeks and plan to vaccinate 100 million Americans by the end of February.
In a press conference on Wednesday, Health and Human Services Director Alex Azar said the FDA would meet on Dec. 10 regarding Pfizer's vaccine candidate and on Dec. 17 regarding Moderna's vaccine candidate. The agency is currently reviewing both for Emergency Use Authorization.
Both companies have been manufacturing doses of their candidate for several months in the hopes of ramping up supply in case of approval. Hundreds of thousands of doses of both doses will be ready for delivery as soon as approval is granted.
Dr. Moncef Slaoui, Operation Warp Speed's chief advisor, stressed that the data from the vaccine trials are "clear" and shows both candidates to be safe and effective.
According to Gen. Gus Perna, the operation's chief operating officer, the Department of Defense is prepared to deliver several million doses of both Pfizer and Moderna's vaccine upon approval. He said Wednesday that states are required to submit their "microplans" for Pfizer vaccine delivery to the Pentagon by the end of the week, and for the Moderna vaccine by the end of next week.
"The states know their populations the best," Perna said.
Both Moderna's and Pfizer's vaccine candidates require two shots that need to be taken 28 days apart, and both vaccines need to be stored at ultra-cold temperatures before use.
Slaoui said Wednesday that government officials expect 20 million Americans to be vaccinated by the end of December and that they project 100 million Americans will be vaccinated by the end of February.
On Tuesday, an FDA panel recommended that health care workers and patients in long-term health facilities would be the first to receive the vaccine. Experts believe the vaccine will be available to everyone who wants it by spring 2021.
Slaoui said Wednesday that government officials expect 20 million Americans to be vaccinated by the end of December and that they project 100 million Americans will be vaccinated by the end of February.
Slaoui added that a one-shot vaccine candidate produced by Johnson & Johnson and a vaccine being developed by AstraZeneca could reach efficacy thresholds by the end of the month and that their approval would improve supply.
Also on Wednesday, Azar stressed that new antibody treatments for COVID-19 are now available to any patients older than 65 who are not in the hospital. He also urged anyone who has recovered from COVID-19 in the last three months to donate their blood plasma to aid in those antibody treatments.
The press conference was held the same day that officials in the United Kingdom granted emergency approval for the Pfizer vaccine.